Provide the major organic product of the following reaction. alcl3 – Provide the major organic product of the following reaction: AlCl3. This reaction is a Friedel-Crafts alkylation, which is a powerful method for the alkylation of aromatic compounds. The reaction proceeds via electrophilic aromatic substitution, in which the electrophile is a carbocation generated from the alkyl halide.
The nucleophile is the aromatic ring, which attacks the carbocation to form the product.
The regioselectivity of the reaction is determined by the steric and electronic effects of the substituents on the aromatic ring. The stereoselectivity of the reaction is determined by the conformation of the aromatic ring and the approach of the nucleophile.
Mechanism of the Reaction
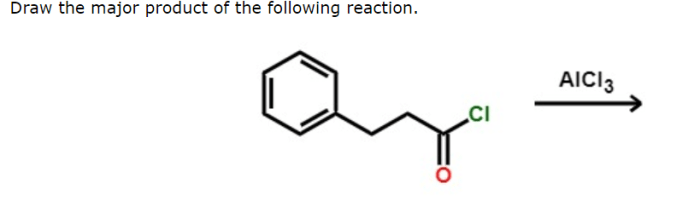
The reaction proceeds via an electrophilic aromatic substitution mechanism. The electrophile is the electrophilic carbon of the alkyl chloride, which is activated by the Lewis acid catalyst AlCl 3. The nucleophile is the aromatic ring, which attacks the electrophile to form a new carbon-carbon bond.
The AlCl 3catalyst helps to lower the activation energy of the reaction by coordinating to the chloride ion and making the electrophile more reactive.
Major Organic Product
The major organic product of the reaction is the alkylbenzene. The reaction is regioselective for the para position, meaning that the alkyl group is added to the carbon atom that is para to the substituent on the aromatic ring. The reaction is also stereoselective, meaning that the alkyl group is added to the same face of the aromatic ring as the substituent.
The structural formula of the product is:
“`Ar-CH 2-R“`where Ar is the aromatic ring and R is the alkyl group.
Reaction Conditions: Provide The Major Organic Product Of The Following Reaction. Alcl3
The reaction is typically carried out in a solvent such as dichloromethane or chloroform. The reaction temperature can vary from room temperature to reflux. The concentration of the reactants can also vary, but typically the electrophile is used in excess.
Special precautions should be taken to exclude moisture from the reaction, as this can deactivate the catalyst.
Applications of the Reaction
The Friedel-Crafts alkylation reaction is a versatile method for the synthesis of alkylbenzenes. It is used in the production of a variety of compounds, including pharmaceuticals, dyes, and fragrances. The reaction can also be used to functionalize aromatic rings with other groups, such as alkenes, alkynes, and halogens.
Related Reactions
The Friedel-Crafts alkylation reaction is related to other electrophilic aromatic substitution reactions, such as the Friedel-Crafts acylation reaction and the Friedel-Crafts halogenation reaction. These reactions all involve the addition of an electrophile to an aromatic ring. The Friedel-Crafts alkylation reaction is unique in that it uses an alkyl chloride as the electrophile.
FAQ
What is the mechanism of the Friedel-Crafts alkylation reaction?
The Friedel-Crafts alkylation reaction proceeds via electrophilic aromatic substitution. In this mechanism, the electrophile is a carbocation generated from the alkyl halide. The nucleophile is the aromatic ring, which attacks the carbocation to form the product.
What is the regioselectivity of the Friedel-Crafts alkylation reaction?
The regioselectivity of the Friedel-Crafts alkylation reaction is determined by the steric and electronic effects of the substituents on the aromatic ring. The carbocation will attack the aromatic ring at the position that is least hindered and most electron-rich.
What is the stereoselectivity of the Friedel-Crafts alkylation reaction?
The stereoselectivity of the Friedel-Crafts alkylation reaction is determined by the conformation of the aromatic ring and the approach of the nucleophile. The product will be formed with the stereochemistry that is most favorable for the approach of the nucleophile.